Spine Implant OEM Manufacturing Solutions
Clinical Significance and OEM Challenges of Spinal Implants
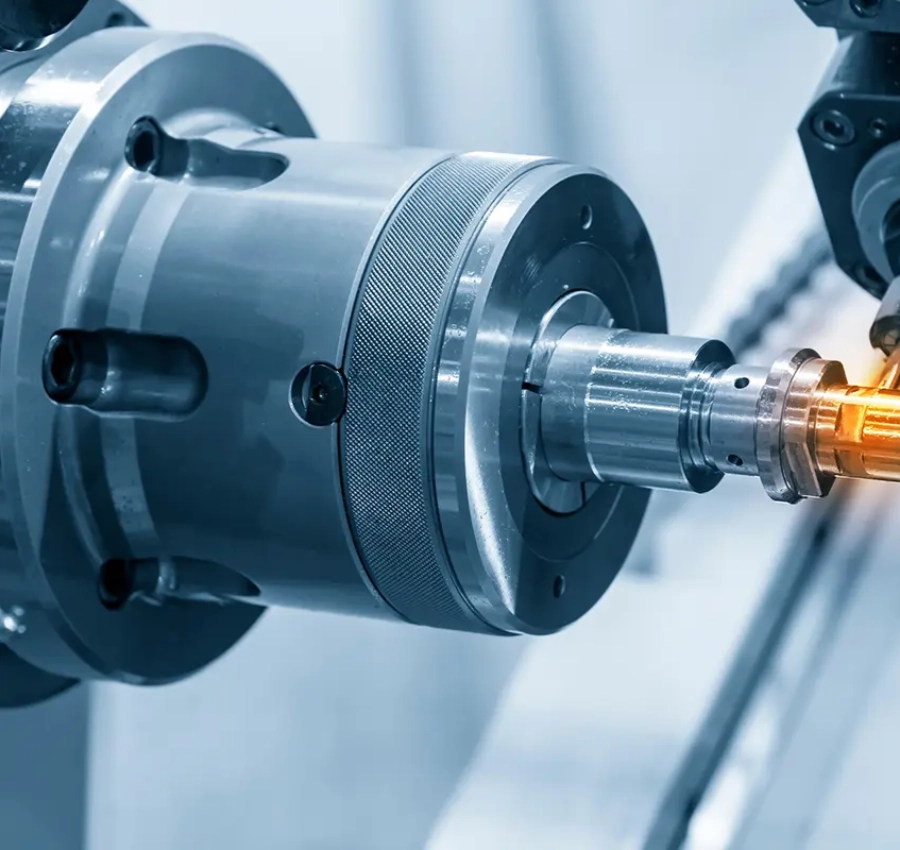
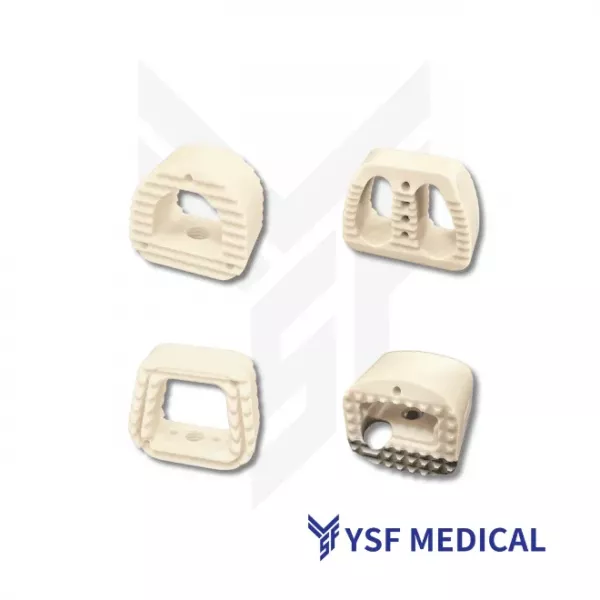
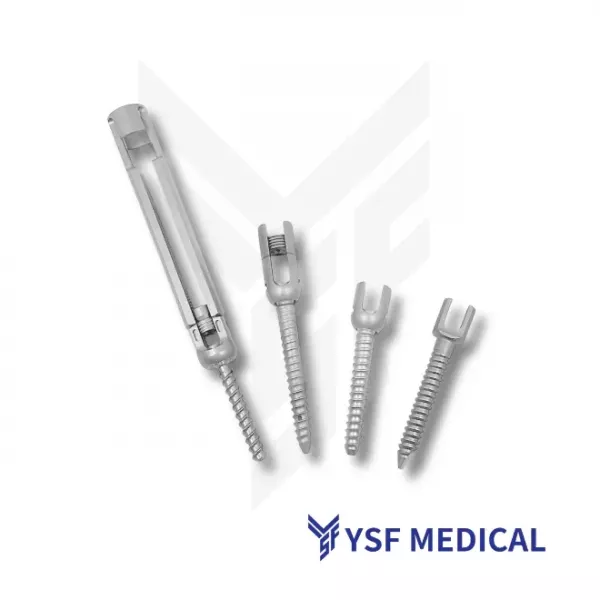
The spine is the central component of the human musculoskeletal system, providing both structural support and mobility. When degeneration, trauma, or disease occurs, surgical intervention is often required to repair and restore spinal function. Spine implants have therefore become essential tools in clinical treatment. Precision devices such as interbody fusion cages, pedicle screws, and spinal fixation plates help surgeons restore spinal stability and enhance patients' quality of life. For patients, these implants represent not only therapeutic solutions but also a vital source of hope for recovery. For surgeons, they serve as essential tools that enable the application of their professional expertise. The success of procedures such as spinal fusion depends heavily on the safety and reliability of these precision-engineered products. These implants require meticulous design, high strength, durability, and biocompatibility. Therefore, for medical device brands, partnering with an OEM capable of delivering quality, efficiency, and regulatory compliance is crucial to ensuring the successful commercialization of spinal implant products.
Deep Collaboration: Your Comprehensive Partner from Prototyping through Mass Production
As an upstream OEM manufacturing partner in the medical device industry, YSF Medical meticulously oversees every stage of the production process. For over 30 years, we have collaborated with numerous international medical brands, recognizing that OEM services are not just about manufacturing execution—they play a crucial role in helping clients reduce R&D risks and accelerate time-to-market. When design drawings reach us, we do more than execute precise manufacturing. Leveraging our extensive industry experience, we offer design-for-manufacturability recommendations to help clients achieve the optimal balance among quality assurance, cost control, and regulatory compliance. This deeply collaborative approach enables us to confront market competition and clinical challenges alongside our partners, establishing ourselves as a trusted, long-term ally.
Quality Commitment: Ensuring Every Product Reflects Trust and Expertise
To ensure that every product complies with international regulatory standards and clinical requirements, we have fully implemented the ISO 13485 medical device quality management system. From raw material control to final product delivery, we have established a comprehensive quality assurance framework with complete traceability. From rapid prototyping and engineering trials to large-scale production, we provide a flexible and comprehensive OEM service process that ensures every design concept is accurately realized. At YSF Medical, we believe an OEM partner is not merely a behind-the-scenes contributor in the industry chain but a driving force advancing the entire medical device sector. Every spinal implant component produced on our lines embodies our commitment to professional quality, precision craftsmanship, and customer trust. We warmly invite you to explore our technical capabilities and service excellence, and to join us in advancing medical device products from concept design to clinical application.
.webp) Contact Us
Contact Us






.webp) CONTACT US
CONTACT US

