
0. Abstract
The medical device industry relies heavily on precision machining, with CNC milling and CNC turn-milling being among the most widely used manufacturing methods. Selecting the appropriate process directly impacts product quality, lead time consistency, and overall production efficiency, especially for regulated medical components where consistency and repeatability are critical.
Many medical device companies have strong product designs but limited in-house manufacturing capacity or access to specialized machining equipment. This limitation is particularly common for high-precision components such as orthopedic implants and surgical instruments. For sourcing, engineering, and quality teams, the decision to use CNC milling machines versus mill-turn machines often depends less on theoretical capabilities and more on managing cost predictability, process risk, and long-term supplier reliability.
This guide outlines the key differences between these two precision machining processes, their optimal applications, and practical cost considerations. Leveraging advanced 5-axis CNC machining and CNC turn-mill capabilities, supported by an ISO 13485-certified quality management system, YSF Medical helps medical device manufacturers make clear and confident outsourcing decisions.
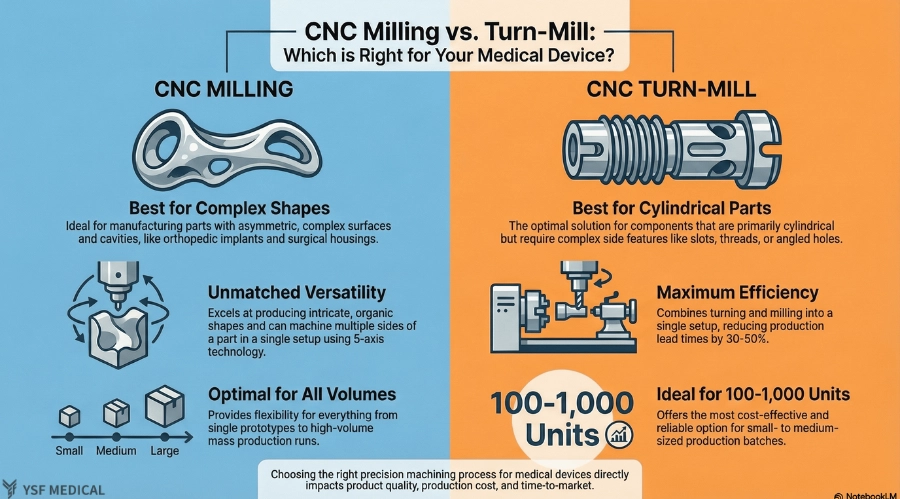
Table of Contents
-
Understanding CNC Milling Machines: The Go-To Solution for Complex Medical Device Geometry
-
CNC Turn-Mill: An Integrated Multi-Process Solution for Medical Device Manufacturing
-
Frequently Asked Questions (FAQ): Medical Device CNC Machining Outsourcing
-
Conclusion: The Right Machining Technology and the Right Partner Make All the Difference
-
YSF Medical: A Reliable Partner for Medical Device CNC Machining
1.Understanding CNC Milling Machines: The Go-To Solution for Complex Medical Device Geometry
1-1 What Is CNC Milling? How It Works
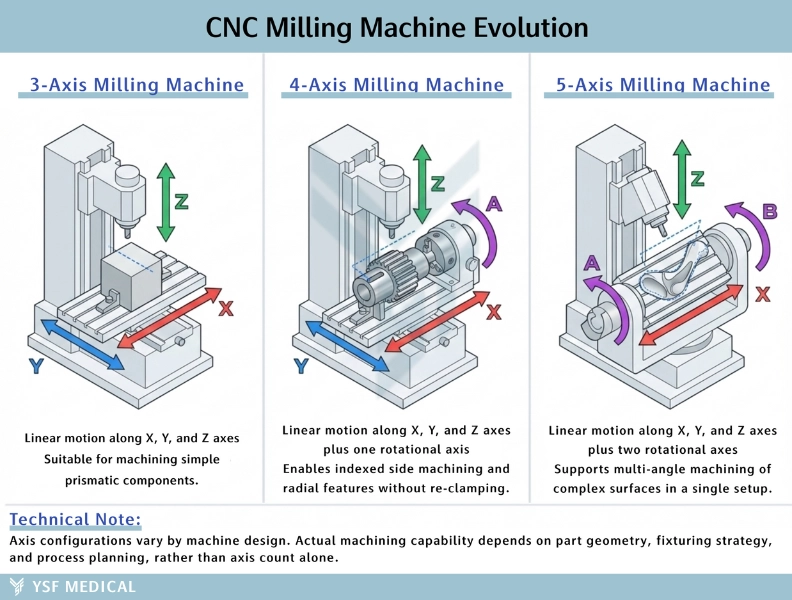
CNC milling machines are available in various configurations based on the number of axes they operate on, ranging from 3-axis models to more advanced 4-axis and 5-axis systems. The primary advantage of adding more axes is simple: the cutting tool can approach the workpiece from a greater variety of angles and orientations. This enhanced maneuverability is particularly valuable when producing the complex, organic shapes required in medical applications.
Consider bone plates, for example, which must conform to the natural contours of skeletal anatomy. Or think about surgical instrument handles that need to be ergonomically optimized to fit comfortably in a surgeon's hand during lengthy procedures. Additionally, implants often require intricate three-dimensional surface textures to promote proper tissue integration. These are precisely the types of components where multi-axis machining capabilities make a significant difference, enabling manufacturers to create geometries that would be difficult or impossible to produce with simpler equipment.
1-2 CNC Milling Machine: Capabilities and Key Advantages
The primary strength of CNC milling lies in its versatility for producing complex geometries. Flat surfaces, curved profiles, slots, pockets, and internal cavities can all be machined with a high degree of precision. For example, internal cavities are often incorporated to reduce weight or enhance ergonomics in surgical instrument housings.
By utilizing 5-axis CNC machining systems, multiple faces of a component can be machined in a single setup. This approach significantly reduces positioning errors and enhances dimensional consistency. For medical-grade components, CNC milling supports tight, repeatable tolerances and surface finishes suitable for both implants and high-end surgical instruments.
These capabilities make CNC milling machines a reliable solution for manufacturing complex structures in medical device applications, from initial development through full-scale production.
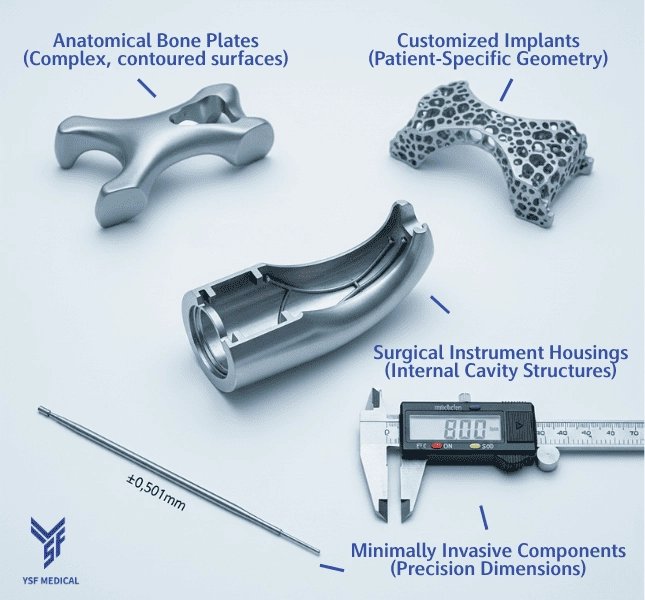
1-3 Common Medical Applications
In orthopedic and surgical manufacturing, CNC milling is commonly used for:
-
Surgical Instrument Housings:
Intricate Internal Features and Ergonomic Exterior Designs -
Orthopedic Implant Bases:
Multi-Angle Surfaces Designed for Accurate Bone Contact -
Patient-Specific Implants:
Customized geometries tailored to individual anatomy using high-speed 5-axis CNC machining. -
Minimally Invasive Instruments:
Thin-walled, precision components where dimensional accuracy is critical.
1-4 Choosing the Right Medical-Grade Materials
Medical devices require a diverse range of materials, each possessing unique machining characteristics. Machining strategies must be tailored accordingly to ensure dimensional accuracy and preserve surface integrity.
-
Titanium (Ti-6Al-4V): Widely used for implants because of its strength and biocompatibility, this material requires specialized tooling and controlled cooling to manage heat generation effectively.
-
316L Stainless Steel: Commonly used in surgical instruments for corrosion resistance, controlled cutting parameters help prevent work hardening.
-
PEEK (High-Performance Polymer): Frequently used for spinal implants, temperature control is critical to prevent deformation.
-
Cobalt-Chrome Alloys: Selected for wear resistance in joint applications, these materials require robust equipment and experienced process control.
2. CNC Turn-Mil: An Integrated Multi-Process Solution for Medical Device Manufacturing
2-1 What Are Mill Turn Machines? An Explanation of the Technology
Mill-turn machines combine turning and milling operations into a single system. A typical CNC mill-turn setup includes a main spindle, sub-spindle, live tooling, and multi-axis control featuring C-axis and Y-axis capabilities.
Unlike standalone milling or turning equipment, CNC turn-mill systems can perform turning, milling, drilling, and tapping operations in a single setup. In medical applications, this integration is especially valuable for components that require threads, angled holes, or side features in addition to rotational geometry.
Multi-axis synchronization allows for precise machining of complex features while maintaining a consistent reference datum throughout the process.
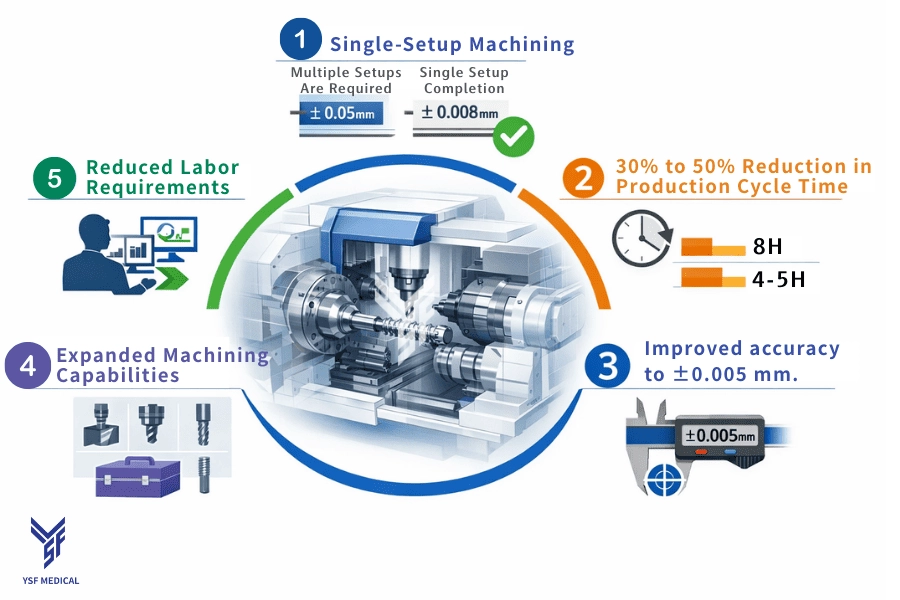
2-2 Five Key Advantages of CNC Turn-Mil
CNC turn-mill offers several advantages for regulated medical components:
1Single-Setup Machining
All features are machined without re-clamping, which reduces cumulative alignment errors and improves concentricity.
2Shorter Lead Times
All features are machined without re-clamping, reducing cumulative alignment errors and enhancing concentricity.
3Improved Dimensional Stability
Machining from a single reference point enhances repeatability and ensures consistent inspection results.
4Expanded Machining Capability
Turning, milling, drilling, and threading are all performed on a single platform, minimizing the need for secondary equipment.
5Reduced Labor Dependency
Higher automation supports stable output with fewer manual interventions.
2-3 Medical Devices Best Suited for CNC Machining Lathe
CNC turn-mill machines are well-suited for components that combine rotational geometry with secondary features, such as:
-
Orthopedic bone screws featuring threaded shafts and lateral locking holes
-
Cylindrical connectors featuring angled or cross-drilled designs
-
Handles for minimally invasive instruments featuring slots or anti-slip patterns4
-
Customized spinal components featuring diverse geometric specifications.
2-4 CNC Turn-Mil vs. Traditional Lathe and Milling
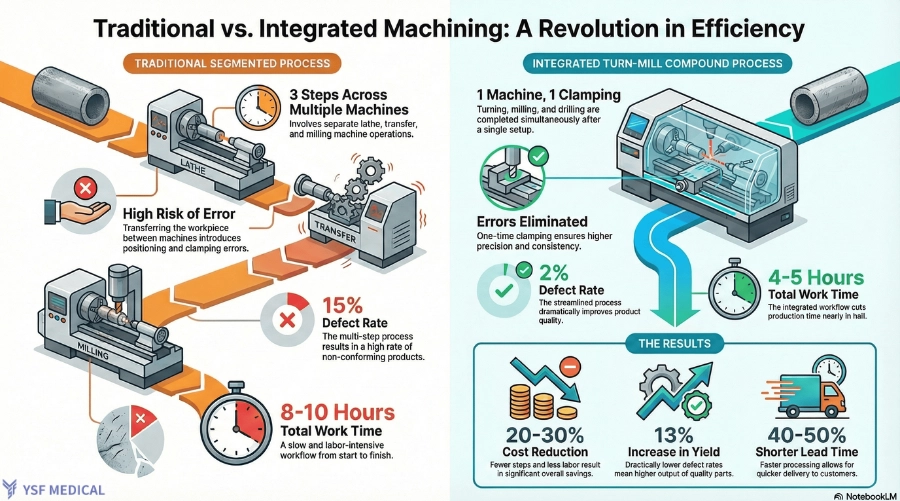
In traditional workflows, cylindrical components are typically machined on a CNC lathe and subsequently transferred to a milling machine for secondary operations.This process introduces several risks:
-
Tolerance Stack-Up Due to Repeated Re-Clamping
-
Logistical Delays Caused by Process Handoffs
-
Increased Quality Variability Due to Manual Setup Dependency
Mill turn machines consolidate multiple operations into a single setup, reducing variability and improving yield. Although machine-hour rates may be higher, the total manufacturing cost is often lower due to reduced scrap, fewer fixtures, and shorter lead times. For this reason, procurement teams evaluate the total manufacturing cost rather than relying solely on the quoted unit price.
3.CNC Milling Machines vs. Mill Turn Machines: Selecting the Optimal Process for Medical Device Manufacturing
3-1 Technical Capability Comparison
CNC milling machines and CNC turn-mill machines are designed to address different manufacturing challenges. CNC milling excels at producing complex, non-rotational geometries such as contoured surfaces, pockets, and internal cavities. It provides greater flexibility in shaping asymmetric parts and is widely used across a broad range of medical device applications.
CNC turn-mill machines, on the other hand, are optimized for components with a cylindrical base that also require secondary features such as side holes, slots, threads, or angled cuts. By integrating turning and milling operations into a single setup, turn-mill machines maintain a consistent datum reference throughout the process, enhancing concentricity and dimensional stability.
From a manufacturing standpoint, the core distinction lies not in precision but in geometry and process integration. Milling prioritizes geometric flexibility, whereas turn-milling emphasizes process consolidation and setup efficiency.
3-2 Selecting the Appropriate Machining Method Based on Product Characteristics
In practice, the selection of a machining method begins with an analysis of the part's geometry. Components characterized mainly by flat surfaces, freeform contours, or internal cavities lacking rotational symmetry are generally best suited for CNC milling machines.
For parts that combine a cylindrical shape with side features such as cross holes, locking slots, or internal threads, CNC turn-milling offers a more efficient and stable solution. Completing these features in a single setup reduces alignment risks and simplifies downstream inspection.
Beyond geometry, functional requirements also play a crucial role. Thin-walled structures, multi-angled surfaces, or features with tight positional tolerances may benefit from multi-axis milling, while shaft-type components with integrated features are often better suited to turn-mill machining. In short, geometry determines the machining method, while functional requirements refine the final process selection.
3-3 Differences in Machining Strategies Between Prototyping and Mass Production
Machining strategies evolve throughout the product lifecycle. During the prototyping stage, flexibility and speed are the primary objectives. CNC milling is often preferred because it enables rapid program modifications, easy tool access, and quick iterations as designs are refined.
As designs stabilize and projects transition into pilot or low-volume production, CNC turn-mill machines offer efficiency advantages for geometries suited to integrated machining. Reducing the number of setups at this stage helps validate process capability and enhances consistency before scaling.
In mass production, machining decisions extend beyond individual processes. Fixture design, automation potential, inspection strategies, and capacity planning become equally critical. At this stage, close collaboration with the manufacturing partner is essential to develop a scalable and repeatable production approach, rather than focusing solely on machine selection.
3-4 Cost-Benefit Evaluation: Looking Beyond the Unit Price
Machining costs should be evaluated from a total cost perspective rather than solely based on the quoted unit price. Factors such as fixture development, setup time, scrap risk, inspection effort, and yield stability all significantly contribute to the overall manufacturing cost.
In regulated medical device manufacturing, processes that minimize setup frequency and manual handling often reduce hidden quality-related costs, improve repeatability, and enhance delivery reliability. Additionally, lead time-related costs are critical in competitive markets, where schedule stability and delivery performance directly impact overall business outcomes.
By adopting a total cost of ownership (TCO) approach that integrates procurement costs, quality risks, and time-related impacts, procurement and engineering teams can make sourcing decisions that prioritize long-term production stability over short-term price optimization.
4. Collaborating with a Medical Device CNC Machining Partner: A Practical Guide from Preparation to Production
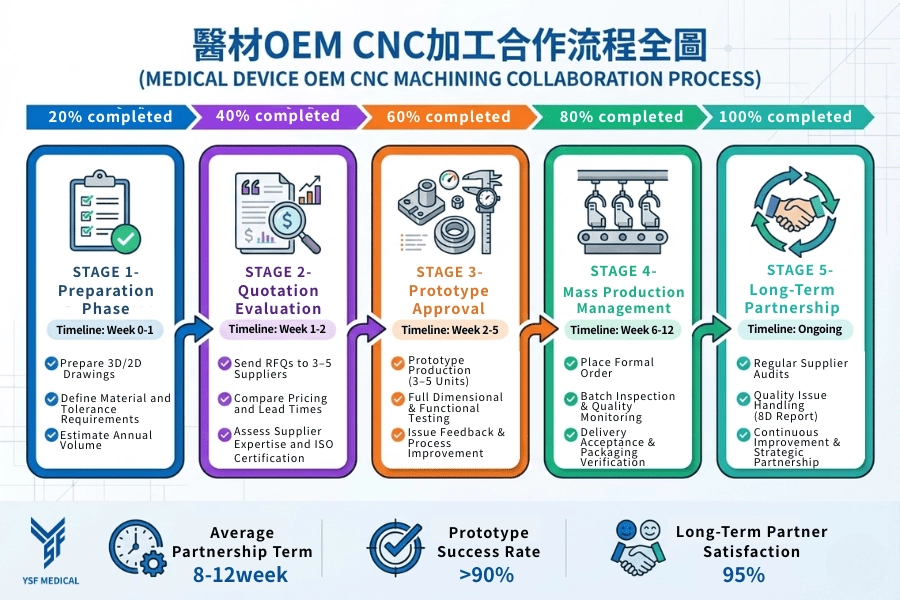
From RFQ Preparation to Stable Production
Selecting the right machining process is only one aspect of a successful outsourcing strategy. Equally important is the collaboration among procurement, engineering, and quality teams with the manufacturing partner throughout the project lifecycle. A structured collaborative approach minimizes misalignment, prevents late-stage changes, and enhances overall sourcing outcomes.
4-1 Preparation: How to Define Your Machining Requirements
Well-defined inputs are the foundation of effective collaboration. For 3D models, the STEP format is recommended to ensure compatibility across CAD systems. Two-dimensional drawings should clearly specify functional tolerances, surface finish requirements, and any critical feature relationships.
Material specifications should include precise grades and relevant standards, such as Ti-6Al-4V ELI (ASTM F136). Tolerances should be based on functional requirements rather than defaulting to the tightest possible values, as overly stringent tolerances increase costs and process risks without enhancing performance.
Additional requirements, such as surface cleanliness, edge condition, or post-machining treatments, should be communicated early. Providing estimated annual volumes and expected ramp-up plans enables suppliers to propose appropriate machining strategies and realistic lead times.
4-2 Evaluating Quotations Beyond Price Comparison
An effective Request for Quote (RFQ) enables suppliers to provide accurate and transparent quotations. When reviewing these quotations, procurement teams should consider factors beyond just the unit price.
Key considerations include lead time assumptions, setup strategies, inspection scope, and process capability. Significant price deviations between suppliers often reflect differences in machining approaches, fixture designs, or risk assumptions rather than pure cost efficiency.
Early technical discussions are a strong indicator of supplier capability. Suppliers who ask specific, practical questions typically demonstrate a clearer understanding of potential risks and are better positioned to support stable production.
4-3 Prototyping and First Article Validation
Prototype builds are designed to validate both the design intent and manufacturing feasibility. Typical prototype quantities consist of a small number of parts, sufficient to confirm dimensions, assembly fit, and functional performance.
The initial article inspection should prioritize critical features and dimensional relationships instead of exhaustive measurement of non-critical characteristics. Functional testing, surface quality assessment, and edge condition verification are essential, especially for surgical instruments and implantable components.
Clear and structured feedback during this stage helps manufacturers refine processes before transitioning to full-scale production. Projects should only advance to larger volumes after confirming design and process stability.
4-4 Managing Production Orders and Ongoing Communication
During production, clear documentation and effective communication are essential. Purchase orders should specify part numbers, revision levels, quantities, delivery schedules, and quality requirements.
Inspection plans and sampling strategies should be aligned with the criticality of the parts and historical process performance. Packaging and labeling requirements must ensure traceability, including lot identification and handling instructions.
Regular communication throughout production enables early identification of potential issues, thereby reducing the risk of delivery delays and quality deviations.
4-5 Long-Term Partnership and Quality Monitoring
Sustainable sourcing depends on consistent supplier performance over time. Regular audits, performance evaluations, and structured corrective action processes facilitate continuous improvement.
In ISO 13485-certified environments, traceability, document control, and corrective action systems play a critical role in maintaining product consistency and ensuring regulatory compliance. Long-term partners evolve from transactional suppliers into technical collaborators who contribute to process optimization and risk mitigation.
Q1: How do procurement teams choose between CNC milling and CNC turning?
The decision typically begins with the part geometry. Components characterized by flat surfaces, complex contours, or internal cavities lacking rotational symmetry are generally better suited for CNC milling. Parts with a cylindrical base and side features such as cross holes, slots, or threads are often more efficiently produced using CNC turn-mill machines.
Production volume further refines the choice. CNC milling offers flexibility for prototyping and small batches, while CNC turn-milling provides greater efficiency and stability for mid-volume manufacturing.
For a clear recommendation, YSF Medical reviews drawings based on geometry, tolerance requirements, and expected volumes. You may send your drawings to sales@ysfbone.com for a complimentary process assessment.
Q2: Is CNC turn-milling more expensive than CNC milling?
On a unit-price basis, CNC turn-mill machining may seem more expensive due to higher equipment investment. However, when factoring in secondary setups, fixtures, and the risks of rework, the total manufacturing cost is often lower.
This cost advantage is most evident in low- to mid-volume programs, where process stability and yield have a more significant impact than marginal differences in machine rates.
If you are comparing quotations, YSF Medical can assist in evaluating total cost drivers beyond the unit price. Please contact us at sales@ysfbone.com for technical support.
Q3: How long does it typically take to transition from prototyping to mass production?
Lead time depends on part complexity, materials, and production capacity. Prototyping typically takes three to four weeks, followed by first article inspection and validation. Production preparation and initial mass production usually require several additional weeks.
Design changes, material availability, and factory capacity can impact schedules, making early alignment essential.
YSF Medical supports customers at every stage to ensure predictable timelines. Please feel free to contact us at sales@ysfbone.com to discuss your project schedule.
Q4: What is the typical minimum order quantity (MOQ) for machining medical devices?
MOQ varies depending on part complexity and process requirements. Prototyping is often feasible with small quantities, whereas pilot and production volumes are influenced by fixture investment and material utilization.
Standardized designs typically allow for lower minimum order quantities (MOQs), while highly customized parts often require larger volumes to achieve cost efficiency.
YSF Medical collaborates with procurement teams to align minimum order quantity (MOQ) expectations with actual production requirements. Contact sales@ysfbone.com to discuss appropriate volume strategies.
Q5: How can I ensure that my design remains confidential when collaborating with an outsourcing partner?
Design confidentiality depends on both contractual and operational controls. Non-disclosure agreements provide legal protection, while controlled access to design data minimizes exposure risk.
Collaborating with an experienced manufacturer enhances protection by leveraging established document control and traceability practices.
At YSF Medical, confidentiality management is fully integrated into our ISO 13485-certified quality system. Please contact us if you would like to review our confidentiality and data control policies.
Choosing between CNC milling and turn-mill machining involves more than simply comparing technical specifications or unit prices. It requires understanding how the selection of the process affects quality consistency, supply chain stability, and the long-term cost of ownership. Making the right choice accelerates development timelines, reduces the risk of rework, and establishes a foundation for scalable production. Conversely, making the wrong choice leads to quality variability, schedule delays, and hidden costs that accumulate over time.
The decision framework is straightforward: begin with part geometry, assess functional requirements, consider production volume, and evaluate the total manufacturing cost—not just the quoted unit price. For components with complex, non-rotational features, CNC milling provides flexibility and capability. For cylindrical parts with integrated side features, turn-mill machines offer efficiency and dimensional stability by consolidating operations into a single setup.
The real leverage comes from making this decision early and in collaboration with your manufacturing partner. Early engagement facilitates better fixture planning, more accurate lead time estimates, and fewer late-stage design changes. It also ensures that the machining strategy aligns with inspection requirements, regulatory traceability, and your organization's risk tolerance.
In regulated medical device manufacturing, process decisions are critical. They impact not only the parts you produce today but also the scalability and repeatability of the programs you develop tomorrow. Invest time upfront to evaluate machining processes with the same rigor applied to design validation, supplier qualification, and regulatory strategy. The result is a more predictable supply chain, improved cost control, and greater confidence in your ability to consistently deliver high-quality products.
With over 30 years of specialized experience in orthopedic and surgical device machining, YSF Medical supports medical device manufacturers seeking predictable results rather than just competitive quotes. We specialize in precision CNC milling, multi-axis machining, and integrated turn-mill operations, all managed under an ISO 13485-certified quality management system.
What sets us apart is our collaborative approach with your teams. Many of our customers encounter common challenges: tight tolerances that push machining limits, designs requiring manufacturability input, and production timelines that demand early alignment between engineering and manufacturing. We address these challenges by engaging early, reviewing drawings for process risks, proposing alternative solutions when necessary, and developing realistic schedules based on actual capacity and capabilities.
Our goal is not to be your sole supplier but to be the partner you turn to when precision, traceability, and delivery reliability matter most. Whether you are prototyping a new implant design or scaling up production of an established surgical instrument, we will collaborate with you to ensure the machining strategy aligns with your broader program objectives.
Ready to discuss your next project?
Send your drawings to sales@ysfbone.com for a complimentary technical review. We will provide practical feedback on process feasibility, lead time expectations, and potential cost drivers. No sales pressure—just straight forward technical input.
This content is provided for reference purposes for professionals in the medical device industry. The technical information and machining insights presented are based on practical experience and publicly available sources.
Medical device manufacturing entails specific technical and regulatory requirements. Machining solutions should be assessed based on individual product designs, intended uses, and relevant regulations. Any tolerance ranges, process parameters, or lead time estimates provided are for reference only and may vary depending on materials, equipment, and production conditions.
Industry technologies and regulatory requirements are continually evolving. While reasonable efforts are made to ensure the accuracy of the information provided, details may change over time. No responsibility is assumed for any direct or indirect consequences resulting from the use of this content.
9. References
American Society for Testing and Materials. (2013). ASTM F136-13: Standard specification for wrought titanium-6aluminum-4vanadium ELI (extra low interstitial) alloy for surgical implant applications (UNS R56401). ASTM International.
https://www.astm.org/f0136-13.html
Engineering.com. (2018, February 12). Precision CNC machining of medical implants.
https://www.engineering.com/precision-cnc-machining-of-medical-implants/
Fictiv. (2023, November 14). CNC materials series: Tips for CNC machining titanium for medical and aerospace industries.
https://www.fictiv.com/articles/cnc-materials-series-tips-for-cnc-machining-titanium-for-medical-and-aerospace-industries
International Organization for Standardization. (2016). ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes. ISO.
https://www.iso.org/standard/59752.html
International Organization for Standardization. (n.d.). ISO 13485 — Medical devices. ISO.
https://www.iso.org/iso-13485-medical-devices.html
JLCCNC. (n.d.). CNC machining in the medical industry.
https://jlccnc.com/blog/cnc-machining-in-the-medical-industry
Kaleidoscope Innovation. (2023, August 17). Rapid prototyping revolutionizing orthopedic device development.
https://kascope.com/rapid-prototyping-revolutionizing-orthopedic-device-development/
Xometry. (n.d.). Medical CNC machining service.
https://www.xometry.com/capabilities/cnc-machining-service/medical-cnc/
Zintilon. (2025, June 18). Precision medical components: CNC machining services.
https://www.zintilon.com/blog/medical-cnc-machining/
© 2025 YSF Medical Enterprise Co., Ltd. | Experts in Orthopedic Implants and Surgical Instruments Manufacturing | ISO 13485-Certified Medical Device Manufacturer | Precision CNC Machining | All rights reserved. Please cite the source when quoting or reproducing any content.
.webp) Contact Us
Contact Us



.webp) CONTACT US
CONTACT US

